Long-term Effects of Buprenorphine & Methadone on Opioid Use
For many individuals in recovery from opioid use disorder, their risk of relapse remains high even after abstinence is initiated and sustained for multiple years.
The two most popular opioid agonists are buprenorphine (brand name Suboxone when combined with naloxone,) & methadone.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
There are dozens of studies suggesting medication-assisted treatment with opioid agonists (medications that stimulate opioid receptors, reducing craving and opioid overdose risk) improve abstinence rates over the short term (for 3-6 months) relative to individuals who receive an inactive, comparison intervention such as case management.
Because of the ongoing relapse risk associated with opioid use disorder recovery, however, research is needed to understand how individuals respond to agonist medication assisted treatment over multiple years.
This important and timely study by Hser and colleagues reported on 5-year outcomes of individuals with opioid use disorder who initially received one of these two types of medication-assisted treatment in a randomized controlled trial.
HOW WAS THIS STUDY CONDUCTED?
This study by Hser et al. analyzed individuals who initially participated in a real-world treatment trial called Starting Treatment with Agonist Replacement Therapy (START). That initial trial was designed to investigate buprenorphine (with naloxone; but referred to here simply as buprenorphine) versus methadone in terms of their safety when prescribed in real-world clinical settings (i.e., a “Stage IV” trial), particularly related to their effects on the liver. Although not a focus of this study, that trial showed no differences in the safety profiles of the two agonist medication-assisted treatments (e.g., liver functioning and adverse health effects; see here).
This long-term follow-up assessed 795 of the original 1269 participants on opioid use and treatment utilization outcomes, and 1080 of the 1269 on rates of death, about 5 years, on average after they entered that initial study. All participants were diagnosed with opioid use disorder (i.e., formerly opioid dependence from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition), and received up to 24 weeks of agonist medication-assisted treatment at a federally licensed outpatient opioid treatment program. These participants, on average, were 37 years old at study intake, and 65% were male, while 73% were White, 9% Black, 11% Hispanic, and 7% other race/ethnicity. Two-thirds were injection drug users.
The goals of this study were to compare death rates as well as opioid use and ongoing treatment participation over time in each of the medication-assisted treatment groups (i.e., whether participants were randomly assigned to receive buprenorphine or methadone in the initial trial). The study also investigated the impact of whether a patient was receiving buprenorphine versus methadone, regardless of their randomly assigned group.
For the sake of clarity, when we add the word “group” after the medication, that means the individual was initially assigned to receive that treatment. If we do not say “group”, that refers to the effect of the medication, regardless of the person’s initially assigned group.
Like other studies that compare two groups of patients, characteristics that were initially different between the groups and might unduly effect the outcomes were adjusted for in statistical analyses, including the clinical site, baseline cocaine use (yes/no), and demographic characteristics.
Opioid use (yes/no) was determined by a drug test at the follow-up and self-reported use in the 30 days prior to follow-up. Participants also self-reported use over the course of the entire follow-up period; researchers used a well-known, validated calendar-aided recall method to help individuals report on days of opioid use per month across this 5 year follow-up, on average.
Treatment utilization was also determined by self-report, and was categorized by agonist medication-assisted treatment (buprenorphine or methadone) or any other options including other medication-assisted treatment for opioid use disorder (e.g., naltrexone, an opioid blocker, and referred to as an antagonist), psychosocial treatment (e.g., individual relapse prevention), or no treatment whatsoever. Of note, researchers used analyses that allowed them to examine the relationship between medication-assisted treatment and outcomes at several points in time – rather than a single trend over time – because different patterns were evident as analyses moved further out in time from the initial trial.
WHAT DID THIS STUDY FIND?
Notably from the Study:
- Rates of death were similar among the medication-assisted treatment groups, as was time to death since entering the study among those who died.
- Compared to the buprenorphine group, a significantly greater percentage of the methadone group were abstinent from illicit opioids at follow-up using a combination of urine toxicology screen and self-reported use during the past 30 days (59% vs. 49%).
- Over the course of the long-term follow-up, the methadone group had a sharper decline in opioid use during, and up to, the 2 year period after study intake.
- The methadone group, on average, declined from about 15 days per month of illicit opioid use to 9, whereas buprenorphine patients decreased from about 15 to 11. By 2 years, however, both groups were at about 9 days of use, and continued to decline to 5 days of use per month by end of follow-up.
- The methadone group also had more days in their assigned treatment during the trial, more months in any kind of agonist medication-assisted treatment over the follow-up (63% of months vs. 52%), and were also more likely to be in treatment at follow-up.
In an average month, 14% of participants were taking buprenorphine, 39% were taking methadone, and 47% were not receiving agonist medication-assisted treatment. Taking buprenorphine and methadone were each associated with similar advantages compared to no agonist medication-assisted treatment (about 8 less days of illicit opioid use per month, on average).
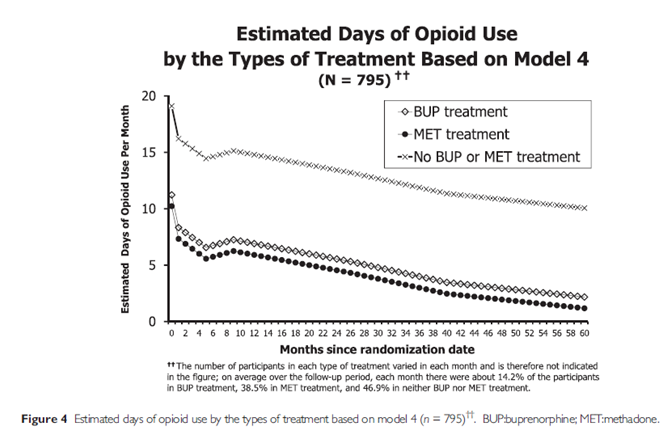
One important observation is that for those on agonist medication-assisted treatment, they are using opioids near 0 days per month by the end of the follow-up period, while those not on agonist medication-assisted treatment, on average, were using illicit opioids about 12 days per month.
Despite similar outcomes for receiving methadone and buprenorphine, overall, the analyses showed that individuals identifying as Whites (compared to non-White) and those reporting cocaine use at study intake had slightly better outcomes in methadone compared to buprenorphine.
This important study builds on the idea that recovery from opioid use disorder is a long-term proposition, for many, but that the majority of individuals are able to achieve abstinence from illicit opioids. Five years after first receiving medication-assisted treatment, about 60% of those who initially were prescribed methadone, and 50% of those initially prescribed buprenorphine, were abstinent based on both negative toxicology screens and self-reported 30-day abstinence.
These findings are quite similar to another long-term follow-up of individuals with non-heroin opioid use disorder who participated in a trial of buprenorphine, the Prescription Opioid Addiction Treatment Study, or POATS. In that study, about 60% of individuals were abstinent 3.5 years after the initial trial – 50% of whom were no longer receiving agonist medication-assisted treatment. About 80% of those on agonist medication-assisted treatment at the final follow-up were abstinent from opioids, compared to only 50% of those not on agonist treatment.
In the study, the percent abstinent for individuals receiving methadone, buprenorphine or no agonist medication-assisted treatment was not reported to be able to compare the two studies directly. However, the potential advantage for agonist medication-assisted treatment vs. not – at least in terms of days of illicit opioid abstinence – is fairly striking with about 8 days per month more abstinent, and reaching almost total abstinence, on average, versus 10 days of use in the month just before the 5-year follow-up. Although it is possible that individuals who are doing well decide to seek agonist medication-assisted treatment, this is very unlikely. More realistic given the dozens of positive results in randomized trials is that receiving agonist medication-assisted treatment helps individuals reduce their opioid use.
One very important difference between these two long-term follow-ups was in the Prescription Opioid Addiction Treatment Study (POATS), individuals with lifetime injection heroin use or any heroin use on 5 days in the past month were excluded from the trial.
On the other hand, Starting Treatment with Agonist Replacement Therapy (START) participants (i.e., from this study summary) averaged 24 days of heroin use and 20 days of injection heroin use in the month prior to treatment intake. Injection heroin use could mark a more severe, and consequential form of opioid use disorder, that would be less likely to remit in the absence of medication-assisted treatment – as was the case for 30% of participants in the Prescription Opioid Addiction Treatment Study.
Indeed, studies show some individuals transition from pharmaceutical opioids to heroin over time, as the disorder becomes more severe, and individuals must switch to the cheaper option (i.e., heroin) in order to continue using. As an example of this greater severity for heroin users, in the Prescription Opioid Addiction Treatment Study, any heroin use in the month before treatment intake (versus none) was a risk factor for continuing to meet criteria for opioid use disorder at the 3.5 year follow-up.
WHY IS THIS STUDY IMPORTANT
The results of this study have important implications for treatment programs & providers, providing insight on the long-term effects of agonist medication-assisted treatment & may inform best practices in treatment programs & funding priorities for policy makers, that indicate advantages of one medication over the other. More specifically, the findings may also speak to under which conditions this advantage holds, as well as to whom any potential advantage applies.
In terms of treatment response, better treatment retention and outcomes for the methadone group versus buprenorphine group are consistent with a previous meta-analysis.
As Hser et al. mentioned, this initial advantage for individuals taking methadone could be due to its greater affinity for opioid receptors, and more reinforcing effects (e.g., craving reduction). From a treatment and recovery perspective, however, this greater reinforcement also increases the potential for the medication to be misused (i.e., not as prescribed), and may be a riskier choice for some.
For example, although not reported directly in this study, in the initial trial, there were nine serious adverse events in the buprenorphine group and eight in the methadone group likely due to study participation. Specifically, one of nine in the buprenorphine group was an overdose from combining the medication with a benzodiazepine, and four of eight in the methadone group were overdose from combining with a benzodiazepine, one from inadvertent methadone overdose, and one death from combining methadone and cocaine.
In addition, while buprenorphine in this trial was administered at the opioid treatment program daily (like methadone), this agonist medication-assisted treatment is more accessible and available because it can be prescribed by physicians in the community who are properly certified. In fact, adding more opioid treatment programs that prescribe buprenorphine, on the whole, would be a far less efficient method of increasing access to buprenorphine relative to increasing the number of physicians that can prescribe the medication.
While methadone may be a more “effective” medication in the short-term, the greater accessibility of buprenorphine & flexibility with which it can be prescribed, suggest it may have greater potential for positive public health impact relative to methadone.
- LIMITATIONS
-
- A key feature of this study was that agonist medication-assisted treatment was compared to all other treatment options, including non-agonist medication-assisted treatment, psychosocial treatment, and no treatment whatsoever.
- In addition, only opioid use over time was reported. More information on other drugs, including alcohol, would help characterize the long-term outcomes of patients receiving agonist medication-assisted treatment. This added information regarding other drugs could be particularly informative given reports in a recent review showing alcohol use is common among those in recovery from opioid use disorder (see here for the RRI monthly research review summary).
- Also, at study intake, one-third of this sample was drinking alcohol, one-third was using cocaine, and one-fifth were smoking marijuana. Whether these other forms of substance use continued, and whether individuals met criteria for drug use disorders apart from opioid use disorder would be helpful to know.
NEXT STEPS
Next steps may include investigating long-term outcomes of other substances in addition to opioid use among those receiving agonist medication-assisted treatment. Also, given that in the Prescription Opioid Addiction Treatment Study (POATS), one-third were abstinent but not on agonist medication-assisted treatment, it may be helpful understand where recovery options overlap, as well as the unique and combined effects recovery management services have on outcomes.
For example, in a prior RRI monthly Recovery Bulletin , we highlighted a study of individuals with opioid use disorder receiving buprenorphine that showed added benefit of participating in the 12-step mutual-help group, Narcotics Anonymous.
BOTTOM LINE
- For individuals & families seeking recovery: With opioid use disorder, chances of getting into and staying in recovery increase if when receiving medications like methadone or buprenorphine – at least specifically in terms of opioid use. More research needs to be done on overall quality of life and functional outcomes, as well as rates of alcohol and non-opioid drug use over time among those with opioid use disorder to be able to make definitive statements about the impact of these medication-assisted treatments, more generally.Precise clinical recommendations are unclear for the appropriate amount of time that individuals with opioid use disorder should take an agonist medication, such as buprenorphine and methadone. Evidence suggests these medications help reduce frequency of opioid use not only during the earliest stages of recovery – during the first 3 to 6 months – but also over the course of the 3-5 years after receiving the medication for the first time.
- For scientists: This well-done study suggests that, among those with opioid use disorder, individuals receiving agonist medications like methadone or buprenorphine will use opioids substantially less often (about 8-10 days per month) than those not on agonist treatment. Because a prior narrative review (see here) reported ongoing alcohol use among those in remission from opioid use disorder – and the impact of this drinking on functioning is less clear – future research should investigate more closely alcohol and non-opioid drug use over time among those receiving agonist medication-assisted treatment.
- For policy makers: Both methadone and buprenorphine appear to help individuals with opioid use disorder reduce illicit opioid use over the long-term, with few differences between the two. Continue to consider developing and implementing policies that increase individuals’ access to these key treatment options.
- For treatment professionals and treatment systems: Both methadone and buprenorphine are helpful medications for reducing illicit opioid use over the long-term (i.e., several years). Although relatively rare events, the risk of overdose, particularly when taken in combination with other drugs like benzodiazepines, remains a possibility that must be considered by prescribers and programs.

