Can cannabis really do all that? Weighing the literature on cannabis-based compounds for mental health disorders
Plant derived and synthesized cannabinoids including tetrahydrocannabinol (THC) and cannabidiol (CBD) are increasingly being prescribed or recommended for medicinal purposes, including for the treatment of mental health disorders and associated symptoms. Currently though, the uptake of these compounds to treat a wide array of mental disorders and their symptoms is outpacing the scientific evidence supporting their use. In this study, the authors assessed the existing scientific evidence for the effects of both natural and synthetic THC and CBD formulations in addressing a range of mental disorders including depression, anxiety, attention deficit hyperactivity disorder (ADHD), Tourette syndrome, post-traumatic stress disorder (PTSD), and psychosis to determine whether, on the whole, there is evidence to support their use for these conditions.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Medicinal cannabinoids, including cannabis-derived and pharmaceutically made tetrahydrocannabinol (THC) and cannabidiol (CBD) are currently getting a lot of play in the popular health media for their ability to treat certain mental health disorders and their symptoms, and are experiencing rapid uptake by individuals seeking THC and CBD products for psychological symptom relief.
At the same time, the scientific evidence for the effectiveness of these compounds for these purposes is controversial. There’s a pressing need to examine and highlight the scientific evidence, or lack thereof, for different types of cannabinoids for the treatment of mental health disorders. The authors of this study sought to address this by conducting a systematic review and meta-analysis (an analysis of many studies at once), which pulled together the existing scientific literature testing the effects of THC and CBD on a range of mental disorders to determine whether there is evidence to support their use in these contexts.
HOW WAS THIS STUDY CONDUCTED?
This was a systematic review and meta-analysis of published studies analyzing the available evidence for the effectiveness and safety, or lack thereof, of cannabinoids for the treatment of a range of mental health disorders. All studies examining a medicinal cannabinoid for treating depression, anxiety, PTSD, ADHD, Tourette syndrome, or psychosis, either as the primary condition or secondary to other medical conditions, were considered in this meta-analytic review.
To identify relevant articles, the authors conducted a systematic search of a number of well-regarded research paper repositories including, MEDLINE, Embase, PsycINFO, the Cochrane Central Register of Controlled Clinical Trials (CENTRAL), and the Cochrane Database of Systematic Reviews via Ovid for studies published from January 1, 1980 to April 30, 2018. To identify ongoing or unpublished studies, the authors also searched ClinicalTrials.gov, the EU Clinical Trials Register, and the Australian and New Zealand Clinical Trials Registry. Additionally, they hand-searched reference lists of papers they identified and topical reviews for potentially relevant articles missed in their paper repository search. No restrictions were placed on language, publication status, or publication type.
Five separate searches were done to identify studies that investigated the efficacy of plant-based (i.e., natural) and pharmaceutical (i.e., synthetic) cannabinoids in treating symptoms of, 1) depression, 2) anxiety, 3) PTSD, 4) ADHD and Tourette syndrome, and 5) psychotic disorders.
The researchers included in their review and meta-analysis all studies examining the use of medicinal cannabinoids in adults aged 18 years or older for the purpose of treating these disorders as a primary condition, or secondary to other medical conditions (such as chronic non-cancer pain). The authors chose to review these specific conditions because they are widely cited as reasons for using medicinal cannabinoids, and commonly have onset in young adulthood and thus have an impact across the lifespan. Both experimental (studies which included some kind of clinical intervention) and observational studies (studies which simply observed individuals already using cannabinoids to manage mental health disorder symptoms) were included in this meta-analytic review.
Studies were considered that examined any type and formulation of medicinal cannabinoid: THC, CBD, combination THC + CBD, cannabis sativa (usually in the form of the bud/flower of the cannabis plant), and other cannabinoids (e.g., tetrahydrocannabinolic acid, cannabidiolic acid, cannabidivarin, and the synthetic-tetrahydrocannabinol formulations nabilone and dronabinol). They categorized these products into pharmaceutical grade THC (with or without CBD), pharmaceutical grade CBD, and medicinal cannabis (i.e., smoked, vaped, or eaten marijuana).
The authors rigorously assessed the quality and risk of bias of each paper they included in their review and meta-analysis to determine how reliably findings suggest actual causality between the intervention/treatment (i.e., THC, CBD) and the outcome (i.e., changes in mental health symptoms). For papers of low quality and/or high risk for bias, we have far less confidence that the study results will translate directly to real world settings.
Analyses were broken down by mental health disorder, the cannabinoid used (pharmaceutical THC or THC + CBD, pharmaceutical CBD, or medicinal cannabis), and the comparison used (active or placebo). For each of these stratified analyses, the authors first pooled the evidence from all eligible randomized controlled trials, regardless of population studied.
After screening, 83 eligible studies were identified (40 randomized controlled trials; n= 3067): 42 for depression (23 randomized controlled trials, including one unpublished study n= 2551), 31 for anxiety (17 randomized controlled trials; n= 605), three for ADHD (one randomized controlled trial; n= 30), eight for Tourette Syndrome (two randomized controlled trials; n= 36), 12 for PTSD (one randomized controlled trial; n= 10), and 11 for psychosis (six randomized controlled trials; n= 281).
WHAT DID THIS STUDY FIND?
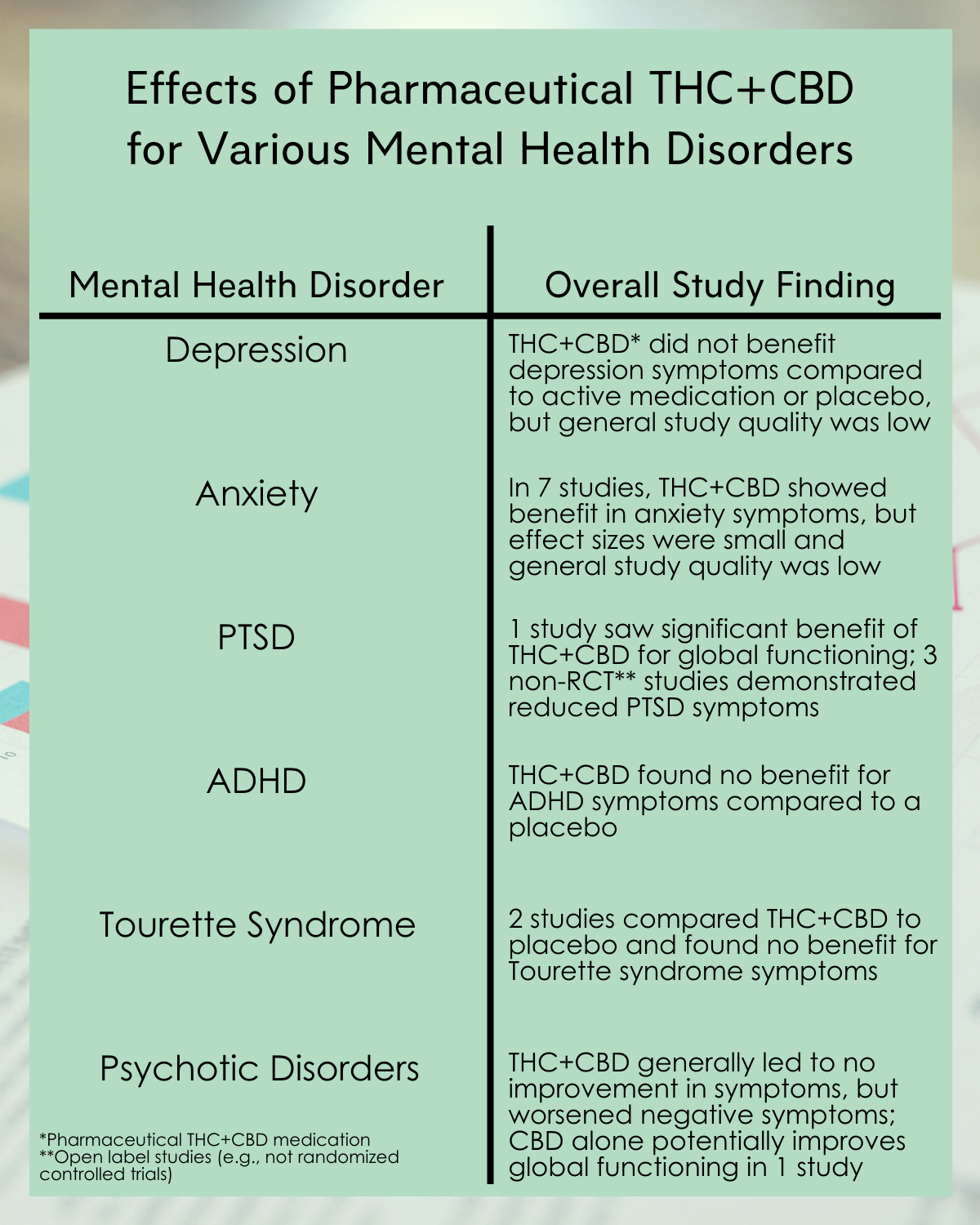
Figure 1.
General observations from the reviewed literature:
- Medicinal cannabinoids were mostly investigated as an adjunct to medical treatments for medical conditions like cancer.
- Randomized controlled trials typically had very small sample sizes (with median sample sizes of 10–39 participants across mental disorders), with short follow-up periods (median trial length 4–5 weeks).
- Across disorders, most randomized controlled trials examined pharmaceutical THC; most commonly in the form of nabiximols and nabilone. The exception was randomized controlled trials of psychosis, which primarily examined pharmaceutical CBD. Few randomized controlled trials examined medicinal cannabis as the treatment.
- In most randomized controlled trials examining depression and anxiety, the cannabinoid was given to treat another medical condition, most commonly chronic non-cancer pain and multiple sclerosis. In studies of other mental disorders, the mental health outcome was the primary target of the cannabinoid.
- Most randomized controlled trials were of unclear or high risk of bias. For instance, many studies did not blind participants or study staff to the treatment participants received.
Depression:
- Pharmaceutical THC + CBD did not significantly improve symptoms of depression compared with either active medications or placebo in randomized trials, including one unpublished study, and generally the quality of these studies was deemed to be low.
- No randomized controlled trials examining CBD alone for depression outcomes were identified.
Anxiety:
- In seven studies (total n=252), pharmaceutical THC + CBD led to significantly greater reductions in anxiety symptoms compared to placebo, though this was a small effect size, with no difference seen in the single, small study that used an active comparison medication. On the whole, the quality was deemed low and risk for bias was high in many of these studies, and thus the confidence in the results from these studies is low. This was in part, because none of the studies included participants with a primary anxiety diagnosis; most included participants with chronic non-cancer pain or multiple sclerosis where anxiety was secondary.
- Two studies examined the effect of CBD in individuals with social anxiety, and did not find an improvement in anxiety symptoms compared to placebo.
- No randomized controlled trials examined the impact of medicinal cannabis on anxiety outcomes.
Post-traumatic stress disorder (PTSD):
- The authors identified a single, small, randomized controlled trial of participants with PTSD; this study did not report either of the meta-analysis’ primary outcomes. Of the secondary outcomes, this study found a significant benefit of pharmaceutical THC + CBD compared with placebo in improving global functioning and nightmare frequency, and no significant effect on sleep quality.
- Three observational studies (i.e., open label with no control group) found reductions in PTSD symptoms in people with PTSD and a co-occurring mental disorder (three involved cannabis and one involved THC extract), while one found that symptoms worsened with cannabis use.
- No studies examined the impact of CBD on PTSD outcomes.
Attention-deficit hyperactivity disorder (ADHD):
- The single, small randomized controlled trial identified for ADHD compared pharmaceutical THC + CBD with placebo among participants with ADHD. No significant effect was seen on ADHD symptoms or global functioning.
- No studies examined the impact of CBD or medicinal cannabis on ADHD.
Tourette syndrome:
- The two small randomized controlled trials identified for Tourette syndrome compared pharmaceutical THC + CBD with placebo among participants with Tourette’s. There was no significant benefit of pharmaceutical THC + CBD compared to placebo on Tourette symptoms or global functioning.
- No studies examined the impact of CBD or medicinal cannabis on outcomes of Tourette syndrome.
Psychotic disorders:
- A single, small randomized controlled trial was identified that reported on the use of pharmaceutical THC + CBD among participants with psychosis. This study found no significant change in positive symptoms (e.g., hallucinations, delusions) but a medium effect size worsening of negative symptoms of psychosis (e.g., blunting of emotions, loss of motivation) with THC + CBD compared with placebo. Of the secondary outcomes, this study also found that pharmaceutical THC + CBD led to a large effect size worsening of cognitive functioning.
- The remaining randomized controlled trials of psychosis examined CBD. Across the one or two studies that reported on primary outcomes, CBD did not significantly improve total symptoms, positive symptoms, or negative symptoms compared with placebo or active comparators. With regard to the secondary outcomes, CBD led to a large effect size improvement in global functioning compared with placebo in the single study reporting this outcome, but did not significantly improve cognitive or emotional functioning.
- No studies were identified that examined the impact of medicinal cannabis on psychosis outcomes.
Adverse events:
- The authors pooled findings for adverse events from all the reviewed randomized controlled trials. Compared to treatment with a placebo, pharmaceutical THC + CBD led to twice as many adverse events, with almost three times the number of participants dropping out of studies.
- No significant differences between pharmaceutical THC + CBD and other cannabinoids were seen with regard to serious adverse events, treatment-related adverse events, or all-cause withdrawals.
- Few randomized controlled trials looked at adverse events and withdrawal effects due to CBD or medicinal cannabis. Of those that did, none found a significant increase in the number of people experiencing adverse events or withdrawing when comparing active dose with placebo.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
To date, this study represents the most comprehensive systematic review and meta-analysis examining the available evidence for medicinal cannabinoids in treating mental health disorders and symptoms.
Findings highlight the notable absence of high-quality evidence where mental disorders are the primary target of treatment, and most evidence is currently derived from studies where mental disorders are secondary to another medical condition, commonly chronic non-cancer pain and multiple sclerosis.
The authors’ meta-analysis found a small reduction in anxiety symptoms associated with pharmaceutical THC (with or without CBD), and one study showed THC + CBD improved global functioning and nightmare frequency in PTSD, however, no effect on depression symptoms, ADHD, or Tourette syndrome was observed. Concerningly, for individuals with disorders characterized by psychosis such as schizophrenia, THC + CBD markedly worsened symptoms. Additionally, THC alone or THC + CBD was associated with much higher likelihood of adverse events than placebo.
A single study of CBD led to large effect size improvements in global functioning among those with a psychotic disorder, but did not significantly improve cognitive or emotional functioning, while two studies on CBD for anxiety found no effect for this compound. No trials with straight CBD have been conducted for depression, ADHD, PTSD, or Tourette syndrome.
The literature for medicinal cannabis (i.e., smoked, vaped, or eaten marijuana) is even more scant, with no studies at the time this meta-analysis was conducted investigating medicinal cannabis for depression, anxiety, PTSD, attention-deficit hyperactivity disorder (ADHD) and Tourette syndrome, and psychotic disorders. This may be in part explained by the challenges of doing clinical trials with medicinal cannabis because of its illegal status with the Federal Government in the United States, the high amount of variability in THC and CBD content in these products, and challenges dosing in a consistent and regimented fashion.
Taken together, the findings of this meta-analysis highlight the major gaps in this literature, particularly a serious lack of high quality randomized controlled trials, and for medicinal cannabis, a lack of any trials whatsoever. Findings to date have largely been negative; in other words, THC and CBD were not shown to be effective for these mental disorders and associated symptoms. Though some open label and observational studies have shown some positive results, and these kinds of studies can be informative, findings from such studies need to be replicated in more rigorous experimental and quasi-experimental studies with an active comparison group (e.g., treatment with cognitive behavioral therapy or anti-depressant medications for depression) so we can get a better sense of whether it’s actually the cannabinoid that’s responsible for any observed benefits, or participants’ expectancies.
Additionally, these findings highlight potential risks associated with THC + CBD for individuals with disorders characterized by psychosis like schizophrenia. Much more research is needed before we can say with confidence that cannabis derivative and medical cannabis have utility for depression, anxiety, PTSD, ADHD, Tourette syndrome, and disorders characterized by psychosis.
- LIMITATIONS
-
- Many clinical trials are under way for cannabis derivatives—particularly CBD for specific psychological conditions—which are of course not included in this meta-analysis. It is unclear how this research will influence the landscape of this literature.
- The authors note that their conclusions are limited by the small amount of available data, small study sizes, and heterogeneity of findings across studies. Small study sizes are of particular concern as effects have been identified to be larger in small studies of medicinal cannabinoids for chronic noncancer pain.
- Additionally, various independent analyses were done and hence might not retain significance if they are adjusted for multiple comparisons.
BOTTOM LINE
- For individuals and families seeking recovery: Findings from this meta-analysis and review highlight the current lack of high-quality evidence for cannabis derivatives like THC and CBD for mental disorders and associated symptoms, and also the risk for adverse events and a worsening of symptoms in disorders associated with psychosis. Though recommendations may change as more research is done, based on the current evidence THC and CBD products and medicinal cannabis should be avoided for the treatment of depression, anxiety, PTSD, ADHD, Tourette syndrome, and disorders characterized by psychosis.
- For treatment professionals and treatment systems: Findings from this meta-analysis and review highlight the current lack of high-quality evidence for cannabis derivatives like THC and CBD for mental disorders and associated symptoms, and also the risk for adverse events and a worsening of symptoms in disorders associated with psychosis. Based on the current evidence, patients should be discouraged from seeking out THC and CBD products and medicinal cannabis for the treatment of depression, anxiety, PTSD, ADHD, Tourette syndrome, and disorders characterized by psychosis.
- For scientists: Findings from this meta-analysis and review highlight the current lack of high-quality evidence for cannabis derivatives like THC and CBD for mental disorders and associated symptoms, and also the risk for adverse events and a worsening of symptoms in disorders associated with psychosis. High-quality randomized controlled trials to properly assess the effectiveness and safety of medicinal cannabinoids, compared with placebo and standard treatments, for the treatment of mental disorders are desperately needed. This evidence is essential before clinical guidelines can be provided about the medicinal use of cannabinoids for these disorders. Also, given the interplay between the cannabinoid system and the neurobiological systems implicated in mood, anxiety, and executive functioning, studies are needed that closely examine adverse events associated with THC and CBD treatments for mental disorders. Further, future cannabinoid studies should investigate how individuals who have not responded well to traditional first-line treatments respond to cannabinoids.
- For policy makers: Findings from this meta-analysis and review highlight the current lack of high-quality evidence for cannabis derivatives like THC and CBD for mental disorders and associated symptoms, and also the risk for adverse events and a worsening of symptoms in disorders associated with psychosis. Cannabis laws are rapidly changing across the United States. Though decriminalizing cannabis use should always be a goal, because of the limited literature speaking to the effectiveness of medicinal cannabis and cannabis derivatives such as THC and CBD, policy reforms about medicinal cannabis should proceed with caution.
CITATIONS
Black, N., Stockings, E., Campbell, G., Tran, L. T., Zagic, D., Hall, W. D., . . . Degenhardt, L. (2019). Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry, 6(12), 995-1010. doi: 10.1016/s2215-0366(19)30401-8

