Does Acamprosate Help Individuals with Alcohol Addiction Maintain Abstinence?
Alcohol use and misuse is a global problem with severe health consequences. Alcohol consumption is the causal factor in more than 200 disease and injury conditions and is responsible for over 3.3 million deaths worldwide every year.
For individuals in recovery from alcohol use disorder, abstinence can be difficult to maintain. Medications are promising options for preventing relapse.
Acamprosate, a medication licensed for use in 25 countries including the United States, works to suppress alcohol cravings by inhibiting elevated glutamatergic neurotransmission.
In Japan, routine clinical practice for alcohol dependence treatment differs greatly from practices in the United States and Europe; patients are hospitalized for 2 months for treatment of withdrawal and post-acute withdrawal symptoms and for stabilization which is then followed by continuing outpatient rehabilitation. The Japanese Acamprosate Study Group conducted a double-blind, randomized, placebo-controlled trial to evaluate the efficacy of acamprosate for maintaining complete abstinence after the 2-month index treatment episode in Japanese patients meeting ICD-10 criteria for alcohol dependence.
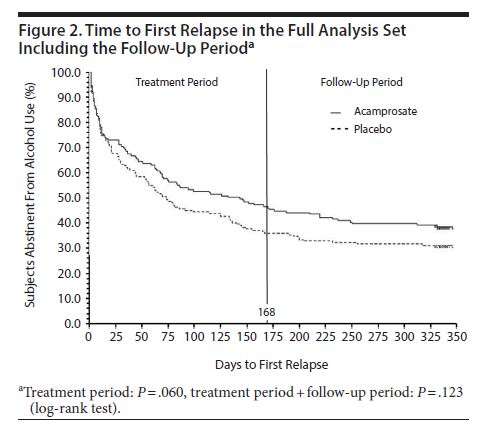
Eligible patients were randomized to oral acamprosate (n = 163) or matched placebo (n = 164) for 24 weeks beginning the day after discharge from inpatient therapy. Participants were followed for an additional 24 weeks after the end of the active treatment phase. Patients received additional therapies including individual or group psychotherapy and referral to self-help groups.
Authors used a rigorous multi-method approach to verify alcohol consumption or abstinence, based on diary entries, interviews with subjects and attendants (i.e., collateral report with someone 20 years or older that lived with the participant), breath alcohol concentration, and laboratory liver functions tests. Participants were 87% male, 52 years old on average, and over half had a duration of alcohol dependence spanning less than 10 years. Patients were not eligible if they had a history of other drug use or misuse, or if they had a psychiatric disorder that required pharmacological treatment.
Regarding the primary outcome, 47% of the acamprosate group and 36% of the placebo group achieved complete abstinence during the 24-week treatment phase.
Source: (Higuchi, 2015)
There were no significant differences between groups in terms of number of days of cumulative abstinence during the treatment period.
Additionally, non-relapse rates were similar (59% for acamprosate and 57% for placebo).
IN CONTEXT
While several medications are approved for the treatment of alcohol use disorder in the U.S., a large multisite randomized controlled trial (RCT) evaluating the efficacy of these therapies found no advantage for acamprosate. This is contrary to results of a meta-analysis of European RCTs which found a therapeutic benefit for acamprosate over placebo for maintaining abstinence.
In support of the evidence from the European randomized control trials (RCTs), results in the current study were similar to those from trials in the United States and Europe where the study drug was initiated immediately after treatment of withdrawal symptoms (versus in Japan where participants were free of withdrawal syndrome and abstinent for the two months spent in inpatient therapy prior to starting the trial).
Thus, acamprosate appears to have an effect despite patients being in a different stage of recovery. This study also employed rigorous methods to ensure reliable data through the use of daily participant diaries and cooperative attendants. The authors reported that 92% of participants attended scheduled study visits and 99% of cooperative attendants attended scheduled visits or were able to be contacted by telephone. Furthermore, biological markers were used for confirmation, providing more confidence in these results.
- LIMITATIONS
-
- However, it is difficult to determine if the superiority of acamprosate holds under all forms of psychosocial therapy engagement. All patients received individual psychotherapy while some also received group psychotherapy, participated in self-help group, or both. When looking at therapy received, those receiving only individual psychotherapy had similar rates of complete abstinence despite receiving acamprosate or placebo. For those receiving all three psychosocial therapies, the difference between complete abstinence rates for the acamprosate and placebo groups widened (66% and 48%, respectively). Without formal analyses, it cannot be concluded if the form of psychosocial therapy received impacts the effect of acamprosate on complete abstinence, presenting an opportunity for further study.
NEXT STEPS
Other outcomes such as percent days abstinent should also be explored as they may provide valuable information on differences between treatment groups.
BOTTOM LINE
- For individuals & families seeking recovery: This study showed the potential for using acamprosate for alcohol use disorders in a setting outside the U.S. and Europe with a different treatment culture and guidelines. The use of cooperative attendants in this study helped keep participants accountable and may have positively influenced their behavior.
- For scientists: This trial is consistent with the conclusions from a Cochrane Review and other randomized placebo-controlled trials and meta-analyses (see here) evaluating the efficacy of acamprosate for the treatment of alcohol use disorder. It is important to determine if these results are replicated in populations with less restrictive exclusion criteria due to the high rates of co-occurring substance use disorders and/or psychiatric disorders among people with alcohol use disorder (see here).
- For policy makers: Acamprosate is a clinically effective treatment option that should be made available to those suffering from alcohol use disorders.
- For treatment professionals and treatment systems: Consider prescribing acamprosate for patients recovering from alcohol use disorder who are motivated to achieve complete abstinence.
CITATIONS
Higuchi, S. (2015). Efficacy of acamprosate for the treatment of alcohol dependence long after recovery from withdrawal syndrome: a randomized, double-blind, placebo-controlled study conducted in Japan (sunrise study). The Journal of clinical psychiatry, 76(2), 181-188.

