Predictive Value of Early Abstinence on Medication Assisted Treatment Outcomes: Buprenorphine-Naloxone in Prescription Opioid Users
Prescription opioid misuse is a growing problem in the U.S. with rates of overdose exceeding those of heroin and cocaine combined.
In light of this epidemic, medication-assisted treatment is an effective option for people who run into problems with prescription opioids.
Determining treatment response/non-response as early as possible is particularly important for those with opioid use disorders given the elevated risks of morbidity and mortality.
McDermott et al. examined the predictive value of initial response to buprenorphine-naloxone (i.e., Suboxone) treatment for 12-week treatment outcomes among individuals meeting DSM-IV criteria for opioid dependence (with prescription opioids as the primary substance used).
Participants were part of the Prescription Opioid Addiction Treatment Study, a multi-site randomized controlled trial consisting of a brief treatment phase (2 weeks of buprenorphine-naloxone, 2 week taper, and 8 weeks of follow-up) and an extended treatment phase (12 weeks of buprenorphine-naloxone stabilization, 4 week taper, and 8 weeks of follow-up) which was offered to those who did not achieve abstinence or near-abstinence in the first phase. Participants were randomized to receive weekly Standard Medical Management (SMM) or SMM and Opioid Dependence Counseling (ODC).
Standard Medical Management (SMM) consisted of medically-oriented counseling during the visit with a physician to adjust dosing, while Opioid Dependence Counseling (ODC) focused on development of relapse prevention skills.
Since over 90% of participants were unsuccessful in the first phase of treatment, only participants (n = 360) from the extended treatment phase were used in this analysis. Participants were 33 years old on average (range: 18 to 64), 58% male, and 91% Caucasian. A majority (81%) did not report past-year non-opioid use disorders.
The authors examined initial response to treatment in four time periods:
- Week 1
- Weeks 1 and 2
- Weeks 1 – 3
- Weeks 1 – 4
The authors operationalized treatment response and success as follows:
- Initial response to treatment: either abstinence or use during every week in a given time period
- Final response to treatment: defined by two outcomes
- Success: abstinence in week 12 (final week of stabilization) and at least 2 of the 3 previous weeks (weeks 9 – 11)
- Abstinence: complete abstinence from opioids in the final 4 weeks of treatment (weeks 9 – 12)
- Positive predictive value (PPV): the degree to which initial abstinence predicted final treatment success or abstinence
- Negative predictive value (NPV): the degree to which opioid use in every week during a given early treatment period predicted an unsuccessful outcome
The tables below describe the POSITIVE Predictive Values (PPVs) of success or abstinence for each initial time period:
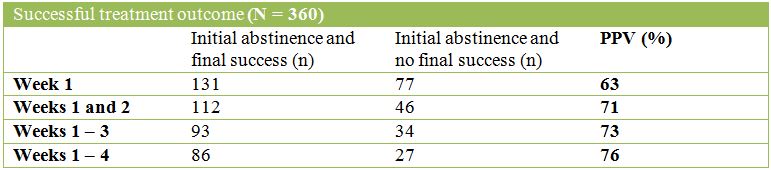
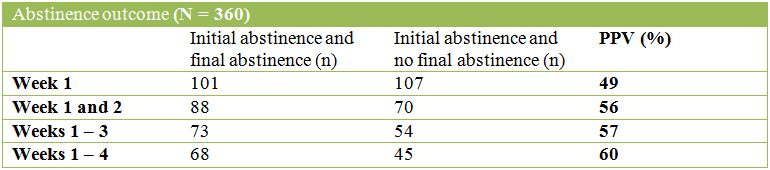
The tables below describe the NEGATIVE Predictive Values (NPVs) of success or abstinence for each initial time period:
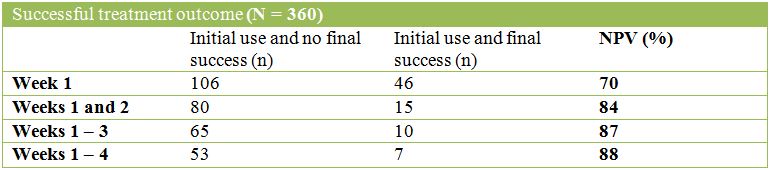
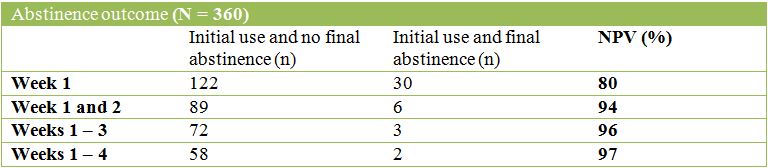
Opioid use during weeks 1 and 2 was a strong predictor of not achieving success at the end of treatment and an even stronger predictor of non-abstinence. For both outcomes, the gains from increasing the initial period were modest with Negative Predictive Value (NPV) increasing by 4 percentage points for the successful treatment outcome and 3 percentage points for abstinence.
IN CONTEXT
In this study, use of prescription opioids in both weeks 1 and 2 after starting buprenoprhine-naloxone treatment is likely indicative of not achieving abstinence or near abstinence by week 12; this may be an optimal time to reevaluate the treatment plan for these patients.
The choice of population for this study (ie., primarily prescription opioid users vs. heroin users alone or a mixed population) is novel and timely. The ability to make an evidence-based clinical modification earlier in the course of treatment can save time and money, but more importantly, could save the life of the patient.
- LIMITATIONS
-
- When interpreting these findings, it is important to recognize that predictive value (ie., PPV and NPV) may not be generalizable to other populations and contexts. For this study testing the predictive value of initial response to treatment, PPV and NPV are dependent upon rates of treatment success. Thus, if the same calculations were performed in a population with different rates of treatment success, the PPV and NPV would change. However, since this two week initial response period is consistent with results from similar studies examining other forms of treatment such as smoking cessation, alcohol, and cocaine treatment, this initial 2-week non-response period may be clinically useful.
BOTTOM LINE
- For individuals & families seeking recovery: This study involved weekly visits with a physician for administration and dose adjustment of the medication. Attend all appointments and communicate openly with prescribers about progress or lack of progress for optimal results.
- For scientists: Evaluation of predictive ability of initial response to treatment through additional statistical methods is necessary for generalizing results. This research question should also be examined in different populations with different primary substances used.
- For policy makers: Requiring continuous monitoring of patients’ clinical response to treatment and adjusting the treatment plan accordingly early in the course of treatment is likely to be cost-efficient and cost-effective as it avoids waiting several months to observe a failed outcome before trying another option.
- For treatment professionals and treatment systems: The first two weeks of treatment may be critical to your patients’ success in achieving abstinence. It is important to be cognizant of their substance use early in treatment through the use of toxicology screens on a regular, consistent basis so changes can be made if needed.
CITATIONS
McDermott, K. A., Griffin, M. L., Connery, H. S., Hilario, E. Y., Fiellin, D. A., Fitzmaurice, G. M., & Weiss, R. D. (2014). Initial response as a predictor of 12-week buprenorphine-naloxone treatment response in a prescription opioid-dependent population. The Journal of clinical psychiatry

